Abstract
BACKGROUND. Standard dosing of decitabine 20mg/m 2 daily given over 5 days in combination with the Bcl-2 inhibitor venetoclax (5-day DEC/VEN), in elderly or chemotherapy ineligible patients with Acute Myeloid Leukemia (AML), induces both a high rate of complete remission (CR/CRi rate 71%) and prolonged survival (median OS 14.2 months). (DiNardo CD et al. Blood. 2019) Extending the duration of decitabine treatment to 10-days (10-day DEC/VEN) may increase efficacy of the combination. In a phase 2 study of newly diagnosed chemotherapy ineligible AML patients aged ≥ 60 years, 10-day DEC/VEN led to a high CR/CRi rate of 89%, with 67% of these being negative for measurable residual disease and a median OS of 18 months. (DiNardo, CD et al. Leukemia. 2020). As a prospective clinical trial comparing efficacy of the different dosing schedule of decitabine in combination with venetoclax is unlikely to be performed, we conducted a single center retrospective analysis comparing 5-day and 10-day DEC/VEN outcomes.
METHODS. We retrospectively studied patients with newly diagnosed AML receiving either 5-day or 10-day DEC/VEN between January 2016 to May 2022 at City of Hope National Medical Center. Patients receiving prior AML therapies including hypomethylating agents were excluded. Responses were evaluated per the ELN criteria (Dohner, H et al. Blood. 2017) Measurable residual disease (MRD) flow cytometry was performed at the University of Washington. Patient characteristics were summarized by frequency and associations between overall response and patient and disease characteristics were tested by Fisher's exact test. Overall Survival (OS) was evaluated by the Kaplan-Meier method and the difference between groups was determined by log-rank test. All statistical analyses were performed using R software.
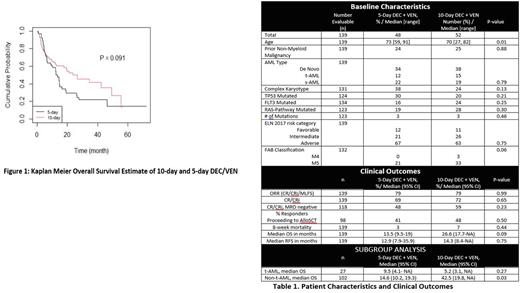
RESULTS. 139 patients were included: 48% (n=67) treated with 5-day decitabine and 52% (n=72) treated with 10-day decitabine. The baseline characteristics of the patients are summarized in table 1. At baseline, 5-day decitabine + venetoclax treated patients were older with a median of age of 73 vs. 70 years (p=0.01). The 5-day and 10-day groups were otherwise well balanced with no significant difference in AML type, ELN risk or adverse molecular features. Rates of CR/CRi, CR/CRi, MRD negativity, and the percentage of responders proceeding to allogeneic stem cell transplant were similar between regimens (Table 1). There was also no difference in 8-week mortality (p = 0.44). With a median follow-up of 12 months for responding patients, the median OS (figure 1) and RFS for 5-day and 10-day DEC/VEN were 14 vs. 27 months, p=0.091 and 12.9 vs. 14.3 months, p= 0.75 respectively. In subgroup analysis, patients without therapy- related AML (non-tAML) receiving the 10-day-regimen had a longer median OS (42.5 vs. 14.6 months, p= 0.03). No other subgroups, including those with high-risk molecular (eg.TP53) or cytogenetic risk factors (e.g. complex karyotype) demonstrated a significant difference between the 5 and 10-day DEC/VEN regimen.
CONCLUSION. Among newly diagnosed AML patients receiving venetoclax in combination with either 5-day or 10-day decitabine, there was no significant difference in response (CR/CRi or MRD negativity) or survival outcomes (OS or RFS). Although a significant difference was not observed, 10-day DEC/VEN led to an increased median OS, which may reach significance with more patients and longer follow-up. Additionally, the 10-day DEC/VEN regimen may benefit certain subpopulations; it demonstrated longer median OS in patients without t-AML. Further analysis to confirm a survival benefit among patients without t-AML receiving either 5-day or 10-day DEC/ VEN is needed and will be presented at ASH.
Disclosures
Koller:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Treadwell Therapeutics: Other: Safety Review Committee. Salhotra:Kadmon: Other: Advisory board meeting ; BMS: Research Funding; Orca Bio: Research Funding. Ali:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Artz:Abbvie: Honoraria; Magenta: Honoraria. Becker:Accordant Health Services (CVS Caremark): Consultancy; Glycomimetics: Research Funding; Pfizer: Research Funding; Notable Labs: Research Funding. Smith:Johnson and Johnson: Current equity holder in publicly-traded company. Nakamura:Helocyte Inc: Research Funding; BluebirdBio: Consultancy; Omeros: Consultancy; Sanofi: Consultancy; Magenta Therapeutics: Consultancy; Kadmon: Consultancy. Pullarkat:Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Lynx: Membership on an entity's Board of Directors or advisory committees. Stein:Amgen: Honoraria. Aldoss:AbbVie: Consultancy, Research Funding; Agios: Consultancy, Honoraria; Amgen: Consultancy; Autolus Limited: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Kite: Consultancy. Ball:Oncovalent: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal